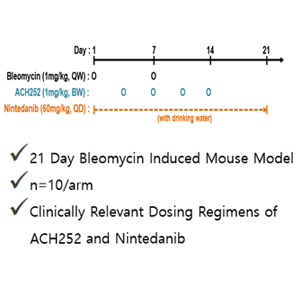
Guaiacol was originally developed for other indications and ACH252 is a liquid-based inhaled formulation of Guaiacol. Our initial animal study showed that guaiacol orally administered is effective for the treatment for IPF. Furthermore, we also conducted study with the potential to be effectively delivered to the lung through commonly used inhaling chamber devices in mice. The inhaled ACH252’s improved pharmacological properties allow it to be delivered directly into the lung tissues and cells, which is expected to reduce both the dose necessary to promote therapeutic effects and whole-body toxicity, compared with oral dosing.
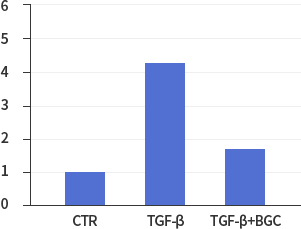
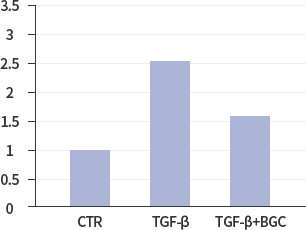
Confirming Efficacy of ACH252 in Cellular Study ModelStudy results showed that lung epithelial cells treated with ACH252(BGC) had a reduction in the activities of fibrotic factors and markers compared with untreated control cells as indicated. Overall ACH252’s efficacy in cellular model study was validated in animal model studies.
-
HIF-1α
-
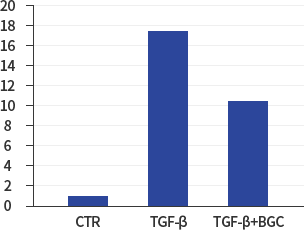
NOX4
-
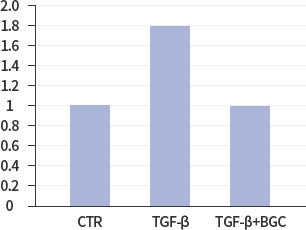
NOX2
-
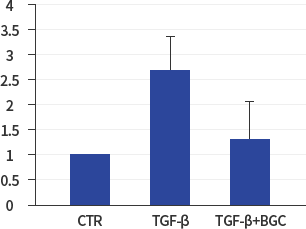
α-SMA
-
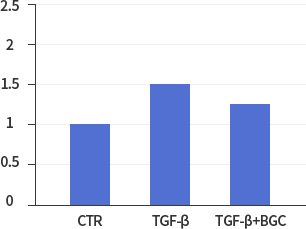
Fibronectin
-
Collagen4
*In vitro lung epithelial cells A549 were treated with TGF-ß (10g/ml), BGC(2μΜ) or both.
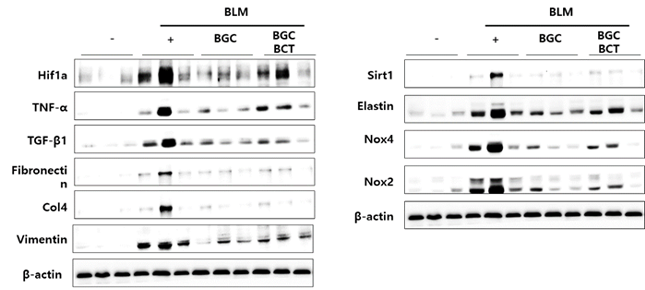
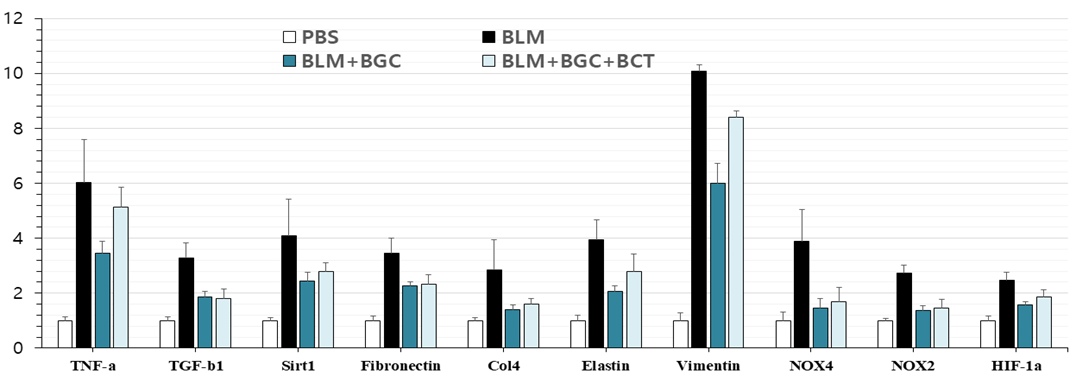
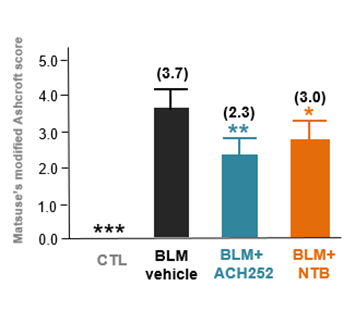
Study results also showed that inhaled ACH252(BGC) attenuated levels of bleomycin-induced fibrotic triggers and makers with statistical significance.
-
Study results showed that mice treated with 1mg/kg of ACH252 via oropharyngeal administration delivered through commonly used inhaler device had a reduction of 38% in the amount of scarred tissue compared with control mice as measured by Trichrome staining and modified Ashcroft scoring while mice treated with 60mg/kg of Nintedanib orally delivered in drinking water had a reduction of 18.9% confirming that ACH252 is an effective anti-fibrotic agent for IPF.
-
-